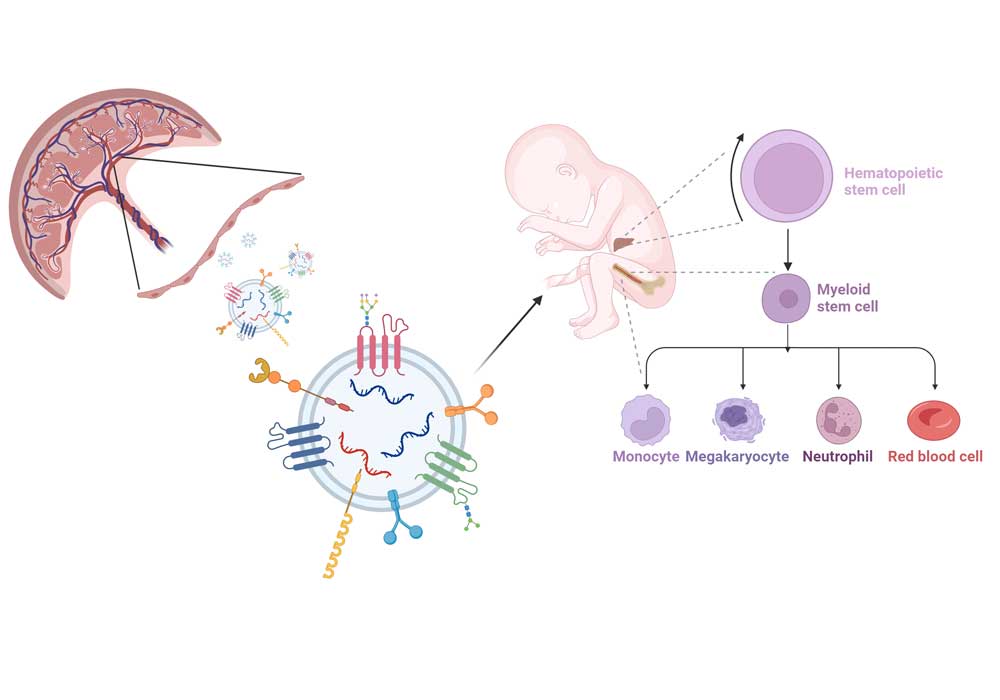
Interaction between Macrophages and Endothelial cells in placental angiogenesis
Macrophages are highly plastic cells. In placenta, they are the main immune cells, but fulfil many more functions in addition. E.g., they promote fetal tolerance in the mother, they regulate tissue homeostasis and remodeling. They are known to interact with trophoblast cells to modulate the extracellular matrix and thereby allow for proper trophoblast invasion. It also has been suggested in the past, that they interact with endothelial cell, remodelling the ECM to allow for angiogenic sprouting and new blood vessel growth.
Here, we investigate how macrophages might influence endothelial cell angiogenesis using both primary Hofbauer cells and feto-placental endothelial cells isolated from placenta. As mono-culture of a single cell types in vitro is a quite artificial set-up, our aim is to establish a co-culture model of endothelial cells and macrophages and use it in functional assays of angiogenesis, to resemble the in vivo situation more closely. As angiogenesis and endothelial function in placenta from pregnancies compromised by pre-eclampsia or diabetes differs from that of healthy pregnancies, investigation of endothelial and macrophage function in such pathologies is essential, too.
Schliefsteiner C, Peinhaupt M, Kopp S, Lögl J, Lang-Olip I, Hiden U, Heinemann A, Desoye G, Wadsack C. Human Placental Hofbauer Cells Maintain an Anti-inflammatory M2 Phenotype despite the Presence of Gestational Diabetes Mellitus. Front Immunol. 2017 Jul 31;8:888. doi: 10.3389/fimmu.2017.00888. PMID: 28824621; PMCID: PMC5534476.
Schliefsteiner C, Ibesich S, Wadsack C. Placental Hofbauer Cell Polarization Resists Inflammatory Cues In Vitro. Int J Mol Sci. 2020 Jan 22;21(3):736. doi: 10.3390/ijms21030736. PMID: 31979196; PMCID: PMC7038058.
Loegl J, Hiden U, Nussbaumer E, Schliefsteiner C, Cvitic S, Lang I, Wadsack C, Huppertz B, Desoye G. Hofbauer cells of M2a, M2b and M2c polarization may regulate feto-placental angiogenesis. Reproduction. 2016 Nov;152(5):447-55. doi: 10.1530/REP-16-0159. Epub 2016 Aug 17. PMID: 27534571.


 Carolin Schliefsteiner
Carolin Schliefsteiner
 Monika Horvat Mercnik
Monika Horvat Mercnik Christian Wadsack
Christian Wadsack
 Michaela Stoiber
Michaela Stoiber